Quality of food supplements – Europe
FoodChain ID and NOW Foods Partner to Showcase Regulatory Leadership
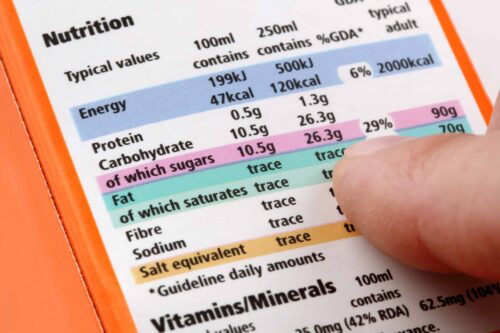
How to create a compliant supplement label for Europe?
What are the requirements for selling supplements online in Europe?
Novel foods and food supplements: understanding the European legal framework
What are health claims?
Service details
Our certification as a professional training organization enables our scientific and regulatory experts to provide personalized, custom training sessions, in the following areas:
Regulatory
- European / national regulatory frameworks:
- Food supplements
- Sports nutrition
- Food for Special Medical Purposes (FSMP)
- Infant nutrition
- Meal replacements
- Fortified foods
- Novel foods
- Legal status of formulations (authorized ingredients, maximum doses, warnings, mutual recognition), labelling rules, procedures of notification or registration.
- Marketing (labels, sales material, leaflets, brochures, instructions, web and in-store): Scientifically and legally acceptable claims in the field of the Regulation 1924/2006 about nutrition and health claims, taking into account the importance of knowing the limits which could lead to a requalification to a drug by presentation or a judgment for misleading advertisement.
- Regulation 1924/2006 (nutritional and health claims): Understanding European Community content, procedures and impositions, deadlines, file constitution, prerequisites, analysis of authorities’ opinions and explanations of the reasons, regulatory compliance, legal penalties.
Scientific
- Nutrition and dietetic basis: structures, roles, sources and needs in macronutrients, vitamins, minerals, functional ingredients (CoQ10 etc.).
- Understanding principal ingredients by application: Indications of the major dietary supplement market segments (energy, weight management, joints, skin, stress/sleep, vision, urinary comfort…) and overview of the principal health benefits and issues with physiological (not pharmacological) improvement targets by key ingredients.
- Studies and efficacy/safety files: requirements and prerequisites, file’s typology, necessary studies in the context of the regulation about claims and nutrivigilance.