By Paula Bertagna
Background and Purpose
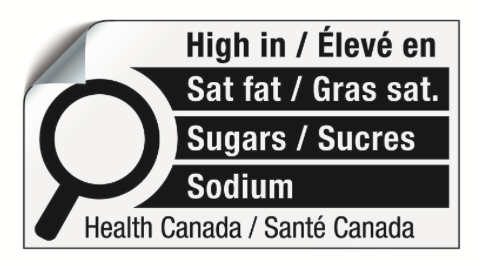
After years of consultations and research activities by Health Canada, the Canadian government amended the Food and Drug Regulations (FDR) in July 2022 to introduce new front-of-package (FOP) nutrition labeling requirements to help Canadians identify products that are high in certain nutrients of public health concern, namely saturated fat, sugars and sodium.
With the rise in diet-related diseases and an increased awareness of the importance of healthy eating, there has been a growing demand for more transparent and understandable nutritional information on packaged foods. High intakes of saturated fat, sugars and sodium are linked to obesity, high blood pressure, and chronic diseases, such as heart disease and type 2 diabetes. The introduction of the “High in” symbol will facilitate effective decision-making by consumers.
Key Components and Transition Period
The FOP nutrition labeling rules consist of the following major parts:
- Nutrition symbol design
- Nutrient thresholds
- Exemptions and prohibitions
- Format specifications (Presentation)
- Restrictions to voluntary health-related representations such as nutrient content claims on prepackaged products that also carry the symbol
The amendments related to FOP labeling are subject to a transition period that ends December 31, 2025. Most prepackaged food products sold in Canada, including those manufactured in Canada or imported are expected to be compliant with the new requirements as of January 1, 2026. Certain foods or types of foods hold either prohibitions or exemptions from displaying the symbol.
Impact and Considerations
The significance of these regulations extends beyond individual health, influencing both public health and the food industry. There is a potential to cultivate better eating habits over time and encourage manufacturers to reformulate products to meet healthier criteria, considering that no one wishes to be the pioneer in introducing labels with such warnings. As the implementation of these regulations unfolds, collaboration between consumers, manufacturers, and regulators will be key to maximizing the potential benefits and ensure a healthier future for all.
Navigating the complexity of product development, staff training and ongoing compliance within a global marketplace can be challenging. FoodChain ID delivers high-level subject matter expertise for consulting based on your business needs, budget and timelines. Click here to learn more and get in touch with our team to discuss your next project.

Paula Bertagna
Consultant, Regulatory Affairs
Paula Bertagna is a subject matter expert in food ingredients, additives, product formulation and labeling compliance, with more than 15 years of Regulatory Affairs experience in the Food Industry. Having lived in Latin America, North America and Asia Pacific, her global journey encompasses over a decade dedicated to the Canadian confectionary & snacks sectors with leading global CPG companies. As a consultant with FoodChain ID, Paula supports food and beverage clients and also collaborates on the monthly production of our Regulatory Trends Digests & Reports. Paula holds a Veterinary Medicine degree from University of São Paulo in Brazil.









